Faster Melting
Calumite Slag increases the melting rate of the batch, thereby allowing furnace temperatures or energy consumption to be reduced
without affecting glass quality. The glass melting process is, in effect, a series of reactions rather than a true melting
process. In the glass tank, a large number of these reactions will be occurring simultaneously, depending on the small local
variations in composition, temperature and atmosphere that will inevitably occur within the furnace. There is a number
of ways in which Calumite Slag is believed to influence the melting process.
As the batch is heated, liquid phases start to form at around 750-800ºC, which then coat the raw material grains, enhancing
the reactions within the melt. This initial liquid phase will include Na2CO3, either from direct melting or reaction with
other batch materials. This liquid reacts with the sand grains, forming sodium silicates, by reactions such as:
Na2CO3 + Si02 = Na20 • SiO2
Na20 • Si02 = NaO • 2SiO2
As the temperature increases, other batch materials will react to become part of the liquid. Limestone and dolomite decompose
at 800-900ºC, through an endothermic reaction releasing CO2 and CaO and MgO that react and form part of
the melt. However, it is known that limestone and CaO are poorly wetted by the primary melt.
The use of Calumite Slag provides between 15% and 40% of the CaO in the new glass from the batch. As the Calumite Slag is glassy and,
therefore, liquid at glass melting temperatures, it is available to take part in the batch reactions at lower temperatures
than the decomposition of limestone and dolomite. This improves the homogenization of the batch and also reduces the endothermic
effect associated with the decomposition of the carbonates.
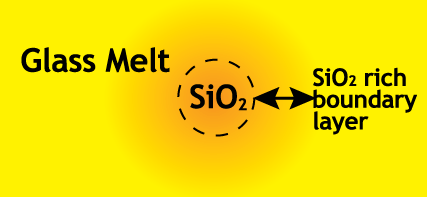
The final stage in the melting process is the dissolution of residual sand grains. As the sand grains dissolve, a SiO2
rich boundary layer will form in the melt around the sand grain. The rate of dissolution will depend on the solubility of
SiO2 in the melt and the rate of diffusion of SiO2 away from the sand grain into the melt. Calumite Slag has a lower
concentration of SiO2 than the glass melt, along with a tendency to wet the sand grains. This results in a SiO2
concentration gradient that encourages dissolution of the sand grains into the melt, thereby increasing the melting rate.
Figure 7.1 - Schematic diagram of sand grain dissolution into the melt
Improved Refining
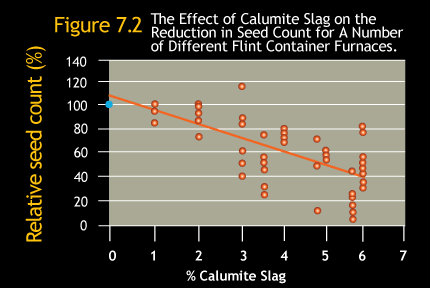
One of the most striking effects of using Calumite Slag is the virtual elimination of seeds and blisters caused by excess residual sulfate,
due to the interaction of Calumite Slag with the sodium sulfate in the batch.
Sodium Sulfate
At temperatures above 1040ºC, the limited solubility of sodium sulfate in the melt causes it to collect at the interfaces
between the melt and unmelted batch or gas bubbles, acting as a surfactant and increasing the fluidity of the melt. The thermal
decomposition of sodium sulfate in the melt occurs at around 1288ºC, producing a stirring action that accelerates the dissolution of
unmelted particles and allows bubbles to rise rapidly through the melt. However the low solubility of sodium sulfate in the final glass
melt composition can result in foam or bubble formation from the decomposition of any excess sodium sulfate, leading to defects in the
final glass.
Effect of Calumite Slag and Sodium Sulfate
In the presence of sulfide, S2-, from Calumite Slag , the decomposition of sodium sulfate occurs at a lower temperature of around
900ºC. The beneficial effects of the interfacial turbulence are therefore experienced earlier in the batch melting process. In addition,
the sulfate - sulfide reaction ensures nearly all the sulfur in the batch is released as SO2, reducing the possibility of
foaming or reboil as a result of excess sulfate.